Coulomb's Law Is Used When Describing
Use bullet points for your answer rather than writing a paragraph. When the charge is 16 x 10¹⁹ C the number of electrons 1.
The value of this constant is.

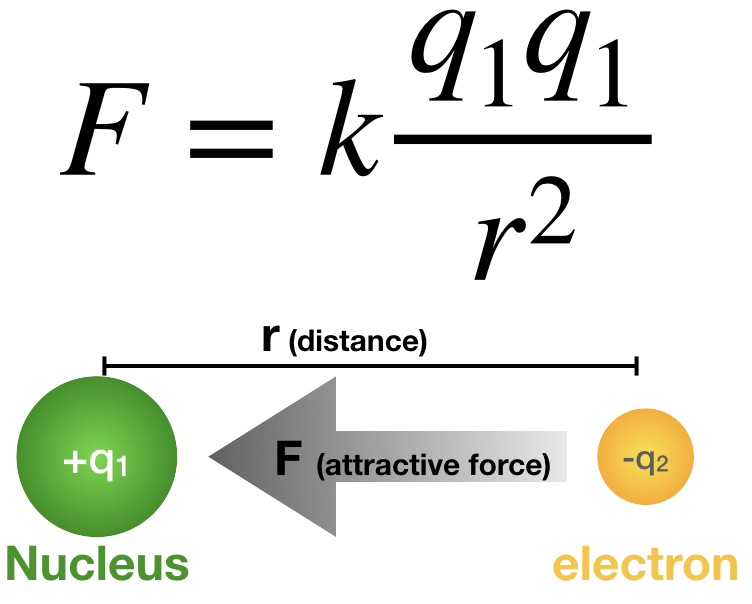
. This law is very important for the development of theory of electromagnetism. The constant of proportionality k is called Coulombs constant. E kq1q2 r.
The magnitude of the electrostatic force of interaction between two point charges is directly proportional to the scalar multiplication of the magnitudes of charges and inversely proportional to the square of the distance between them The force is along the straight line joining them. If they have different signs the force between. Types of Interactions HS-PS2B1.
In equation form Coulombs law can be stated as. Coulombs law was discovered by Charles-Augustin de Coulomb in 1785. The motion of charges The force between charges The proportion between charges The charge of an object.
It quantifies the amount of force between two stationary and electrically charged particles. Solution for Describe the Coulombs law. Derive One Coulomb Charge Value.
Combine that with what you have learned in this activity to complete Coulombs law below. First week only 499. Newtons Law of Universal Gravitation and Coulombs Law provide the mathematical models to describe and predict the effects of gravitational and electrostatic forces between distant objects.
To define Coulombs Law or Coulombs inverse-square law it is an experimental law of Physics. Coulombs law explains the relationship between distance magnitude of charge and force of attraction oppositely charged particles. Coulomb constant k 90 109 Nm2C2.
He used a torsion balance to measure the repulsive and attractive forces between. Here K or k e is Coulombs constant k e 8988 10 9 Nm 2 C 2 1 q 1 and q 2 are the signed magnitudes of the charges and the scalar r is the distance between the charges. If the charges have the same sign the electrostatic force between them is repulsive.
The Coulombs law and the friction factor law are used to describe friction behavior between tools and workpiece materials. Chemists most often use the law in a modified form to calculate the energy of interaction E between the charges. Use Coulombs Law and principles of atomic structure to answer these questions.
Coulombs law states that. This states that the force between two electrically charged particles is proportional to the charges and inversely proportional to the square of the distance between the two particles. Coulombs law states that the magnitude of the force of attraction or repulsion between two point charges is directly proportional to the product of charges and inversely proportional to the square of the distance between them.
Coulombs law is an equation that relates the electrostatic force between two objects to their distance and charge. Charles Coulomb observed that when two electric charges are placed close to each other they experience a force. See What is the Coulombs law.
K 899 10 9 N m 2 C 2. The greater the force of attraction between a negative electron and the positive nucleus of an atom the greater the amount of energy required to separate them thus greater ionization energy. On Solving we get the value of 1 Coulomb charge as 625 x 10¹⁸.
In SI units the constant k has the value k 899 10 9 N m 2 C 2. Coulombs law also known as Coulombs inverse-square law is a law of physics that defines the amount of force between two stationary electrically charged particles known as the electrostatic force. Noting that like charges repel each other and opposite charges attracting each other Coulomb measured the force between.
The electric force present between the charged bodies at rest is conventionally referred to as a Coulomb force or electrostatic force. Hence the law and the associated formula was named after him. The unit vector points directly from the charge toward.
Coulombs law states that the force of attraction or repulsion acting between the two charged particles is directly proportional to the product of the magnitude of the two charges and inversely proportional to the square of the distance between them. Many students can do the math involved with Coulombs equation but may have little comprehension of its significance. F kq1q2 r2.
This formula gives us the magnitude of the force and we can get the direction by noting a positive force as repulsive and a negative force as attractive. Coulombs law is a law describing the interactions between electrically charged particles. Now if the charge is 1 C then the number of electrons will be 116 x 10¹⁹ C.
The direction of the force is along the line joining the centers of the two objects. In chemistry we usually write the formula as. Lets say an electrical circuit carries a charge of the magnitude 16 x 10¹⁹ C.
Where F is the force q1 and q2 are the charges r is the distance between them and k is a proportionality constant. Start your trial now. The force is along the straight line joining the two charges.
This equation is known as Coulombs law and it describes the electrostatic force between charged objects. Where Q 1 represents the quantity of charge on object 1 in Coulombs Q 2 represents the quantity of charge on object 2 in Coulombs and d represents the distance of separation between the two objects in meters. Coulombs law is an experimental law published in 1785 by French physicist Charles Augustin de Coulomb.
COULOMBS LAW The electric force or Coulomb force between two electrically charged particles is equal to 131 We use absolute value signs around the product because one of the charges may be negative but the magnitude of the force is always positive. 24-2 Coulombs Law and Gauss Law Although we work with the familiar quantities volts amps and watts using MKS units there is a price we have to pay for this convenience. In MKS units the constant K in Coulombs law is written in a rather peculiar way namely K 1 4πε 0 ε 0 885 1012 faradsmeter 3 and Coulombs law is written.
Two positively or two negatively charged particles repel each other whereas two opposite charges attract each other. What is the difference between the Coulombs law is valid and the friction factor law is the value of Coulombs friction coefficient the. In activity A you found that the electrostatic force between two objects is proportional to the product of their charges.
The symbol k is a proportionality constant known as the Coulombs law constant.

Coulombs Law By Tutorvista Team Issuu

No comments for "Coulomb's Law Is Used When Describing"
Post a Comment