Explain Why Methyl Cation Has a Different Three Dimensioal
In triphenyl methyl carbocation positive charge can be deiocalized in all the 3 ring system. Hintone is trigonal planar and the other is tetrahedral.
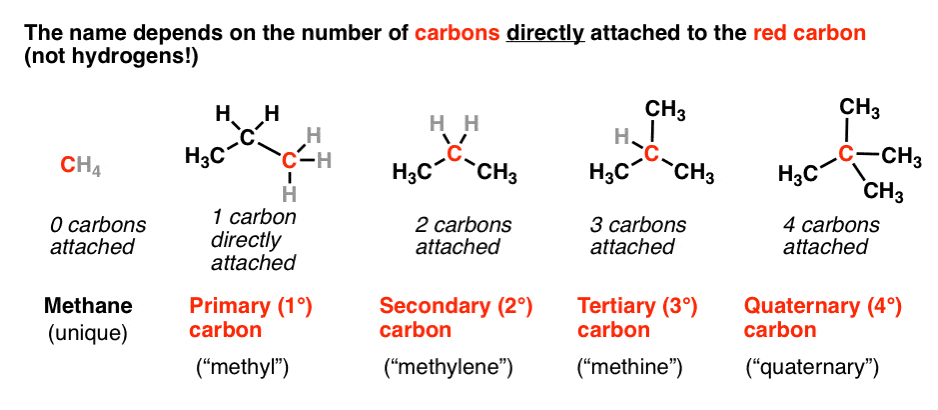
Primary Secondary Tertiary And Quaternary In Organic Chemistry
For example by using solid and dashed wedge formula the 3-D image of a molecule from a two-dimensional picture can be perceivedIn these formulas the solid-wedge is used to indicate a bond projecting out of the plane of paper towards the observer.

. First from these bond angles and Coulsons Theorem ref_1 ref_2 we can determine that the C-H sigma bonds are s p X 22 hybridized and the C-C sigma bond is s p X 17 hybridized. The three-dimensional 3-D structure of organic molecules can be represented on paper by using certain conventions. For example by using solid and dashed wedge formula the 3-D image of a molecule from a two-dimensional picture can be perceivedIn these formulas the solid-wedge is used to indicate a bond projecting out of the plane of paper towards the observer.
While the monopositive cation of helium is the only species of that element that has an odd number of electrons. 9 Which of the above species is a better electrophile. The orientation of the methyl ion fragment is effective only if the dipole-induced orientation occurs in a time shorter than the thermal rotation period of the molecule 01 ns and if the electric field is strong enough.
The three-dimensional 3-D structure of organic molecules can be represented on paper by using certain conventions. What difference can the replacement of one H by methyl make. The H-C-H bond angle in ethene is ca.
A pyramidal structure seems reasonable for the methyl anion however the molecule is not a perfect tetrahedron. Structure and Function 5th Edition - K. To answer this question good three-dimensional drawings are surely necessary.
Solution for Which of the following explains why methyl anion has a pyramidal geometry while methyl cation is planar. 410 və-SEP-ər is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers Ronald Gillespie and Ronald Nyholm.
Rangeli sialidase with two different mechanism-based inactivators at 19 and 17. Important Points To Remember. This is due to the fact that the methyl ion screens the charge of the proton with the CH bond.
Predict the approximate bond angles in a. 117 degrees and the H-C-C angle is ca. 3 Predict the two products you would isolate from the following reaction.
Up to 256 cash back Explain why the methyl cation CH3 is less stable than the cation CH2Cl through. A The ethyl cation 1 is more stable than the methyl cation 2. Valence shell electron pair repulsion VSEPR theory ˈ v ɛ s p ər v ə ˈ s ɛ p ər VESP-ər.
C The simplest organic carbanion is CH 3 which has a trigonal pyramidal structure with an sp 3 hybridized carbon that has a lone pair of electrons. Newman projections are probably the best way to look at the ethyl cation so draw one looking from the methyl group toward the. There are two reasons that combine to explain this angular deformation in ethene.
The orbitals have density at the positively charged nucleus thus a negatively charged electron would be more stable lower in energy in an orbital with more s character. A carbanion is a nucleophileAn electron-rich species that has a. Because it has a strong tendency to share.
The D 3 isotope effect for the transfer of methyl cation from vacuum to water evaluated as an average over 40 solvent configurations each a locally relaxed snapshot from a. Here we present the three-dimensional structures of the covalent glycosyl-enzyme complexes formed by the T. 1 Using both words and pictures explain why ethyl cation is more stable lower in energy than methyl cation 2 Describe hyperconjugation in your own words.
The methyl anion CH 3 has a structure that is similar to NH 3 with its lone pair of electrons but it has a much stronger tendency to share its lone pair with another atom or molecule. The distribution is centered isotropically around the center of mass at far lower velocities compared to the product ions from the other two reaction pathways which are centered at the neutral beam velocity see Fig. Ions which are formed by loss of an electron are termed as cation whereas ions which are formed by g.
This is because one 2s orbital and three 2p orbitals in the valence shell of carbon combine to form four sp 3 hybrid orbitals which are of equal energy and shape. Catalytic H20 4 Calculate the degrees of unsaturation for compounds with the following molecular formulas. Triphenyl methyl cation is very stable due to resonance.
B The methyl radical CH 3 is a radical that like the carbocation is trigonal planar and an electrophile. 8 Explain why CH3 methyl cation has a different three dimensional geometry to CI-13- methyl anion. It is also sp 2 hybridized but there is a single electron in the unhybridized p orbital.
Further four H atoms also use these four sp 3 hybrid orbitals of carbon to form C-H sigma bonds which ultimately leads to the formation of the methane molecule. The most abundant reaction product is the methyl cation CH 3. Vollhardt Neil Eric Schore All the textbook answers and step-by-step explanations.
The HOMO energy decreases upon. Analysis of the kinetic energy distribution reveals two. The hydrogen atom has only one electron and so is a radical.
Causing the lowering of energy and making the carbocation very stable. Primary carbocations have a maximum of 3 σ-bonds capable of hyperconjugation secondary a maximum of 6 tertiary a maximum of 9 hence why the tertiary is the most stable cation out of the simply alkyl groups the allyl and benzyl systems in the diagram above are stabilised through delocalisation of the charge onto the pi system. The hydrogen cation has no electrons while the hydrogen anion and the helium atom each have a pair of electrons and so all of these species are not radicals.
Acids and Bases Polar and Nonpolar Molecules Organic Chemistry. A methyl anion has two more electrons than the cation. For unlimited access to Homework Help a Homework subscription is required.

Ak Lectures Methyl Cation Methyl Anion And Methyl Radical Intermediates

A Methyl Group Is An Alkyl Derived From Methane Containing One Carbon Atom Bonded To Three Hydrogen Atoms Ch3 In Formulas The Group Is Often Abbreviated M

What Is Methyl Cation Methyl Anion And Methyl Radical Organic Chemistry Digital Kemistry Youtube
No comments for "Explain Why Methyl Cation Has a Different Three Dimensioal"
Post a Comment